Zone 2 cardio: All hype or the key to fighting cancer?

Wind Breaker - [Part 3] Ep. 74
Zone 2 cardio is all the rage these days, but does it have any benefit other than making you faster? I wanted to run a simulation to find out if the effects of zone 2 cardio would help you contain the spread of tumor cells.
Cancer is known to grow in acidic environments, such as those created by high concentrations of lactate in the blood. But what could zone 2 cardio really do to help with that? Enter the mighty mitochondria.
The Mighty Mitochondria And Lactate Clearance
We all studied in high school that the mitochondria are the powerhouse of the cell. But it's been a while since I opened a biology textbook, so let's recap a little bit. The mitochondria's job is basically to produce ATP (Adenosine Triphosphate), which for our intents and purposes is just "energy". So what are some of the ways it can create energy? The most common way is glycolysis, which is the process where glucose from your blood enters the cell, gets converted to pyruvate, which gets converted to Acetyl-CoA, which then enters the Krebs cycle and eventually creates ATP. Now, a byproduct of this process is lactate. In regular individuals, this lactate enters the blood and stays there. However, in really fit individuals, like athletes, this lactate can be shuttled back into the mitochondria with special transporters called MCT1 to create even more ATP!
So how does this all relate to cancer cells? Well, cancer cells are wasteful. Like regular cells, they also use glucose as an energy source, but they produce a lot more lactate than regular cells, and this lactate is exported to the blood. In fact, it aids in the creation of acidic micro environments that cancer cells thrive in. So my question is, can these muscle fibers with high concentrations of mitochondria, created through prolonged zone 2 cardio, help clear lactate in the blood produced by these cancer cells? In particular, can these tissues have a non-local effect? i.e., if a tumor grows in, say, the liver, can muscle fibers in the legs help contain the spread of this tumor by clearing lactate from the blood?
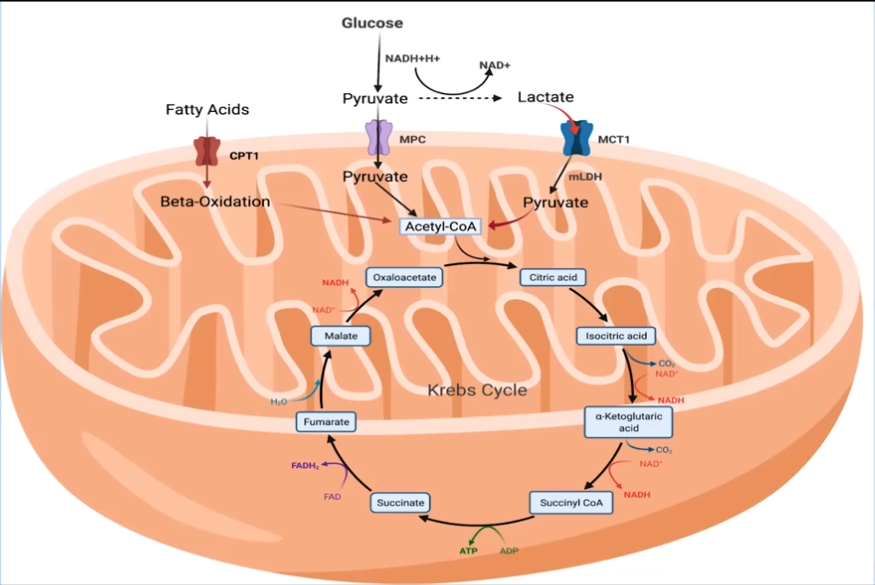
Source: 201 - Deep dive back into Zone 2 Training | Iñigo San-Millán, Ph.D. & Peter Attia, M.D https://www.youtube.com/watch?v=-6PDBVRkCKc
So how do we model this?
I used a cellular automaton to model this process. I used a 2D grid where
each cell represents a biological cell. Each cell can be in one of the
following states:
-
Athlete
An Athlete cell here refers to a cell that has a high concentration of MCT1 transporters, or highly efficient mitochondria that have the capability to clear lactate.
-
Normal
These are normal cells that use glucose as a fuel source but do not have the capacity to turn lactate into fuel. They do not consume as much glucose as athlete cells but do produce more lactate.
-
Cancer
These are cancer cells. They consume a lot of glucose and produce a lot of lactate, aiding in the production of acidic microenvironments where cancer thrives and grows.
Nutrient diffusion
Now that we have set up our grids, we need to figure out how to disperse nutrients, glucose in our case, to these cells. We will model a steady stream of blood supply to the cells, i.e., in discrete time, glucose is supplied to our cellular grid. In the spirit of making the simulation somewhat realistic, I used a convolution matrix to model nutrient diffusion, the process in which nutrients go from high concentration areas to low concentration areas. I used the following kernel
| 0.05 | 0.1 | 0.05 |
| 0.1 | 0.4 | 0.1 |
| 0.05 | 0.1 | 0.05 |
This means a cell keeps 40% of the glucose to itself, shares 10% with its left, right, top and bottom neighbors, and 5% with its diagonal neighbors.
Nutrient consumption and lactate production
Now that nutrients have been diffused, the cells need to consume them. Each cell type has a hardcoded glucose consumption rate. All cells then produce lactate, and athlete cells consume this lactate at a specified consumption rate.
Cancer spreading phase
After all this, we check each cell's Moore neighborhood to see if there are any cancer cells and if the lactate levels have reached a toxicity threshold (also hardcoded). If so, we simply turn that cell into a cancer cell.
Simulation time!
Thanks to the great wonders of WebAssembly, I was able to get my Python code running in the browser, and with a few small tweaks to make it interactive. To start the simulation, pick a ratio of athlete to normal cells and click the start button. The idea is that the fitter you are, the more of these athlete cells you have. Then, introduce a tumor cell in any position by simply clicking on the grid and watch how it spreads! Do tumors that spawn in normal tissue spread as fast as when there are a vast number of athlete cells in the grid?
Pyodide is loading...
Pyodide is loading...
Results
For a set ratio of athlete cells to normal cells, I ran the experiment 5 times to get a sense of the variance in how fast tumors would spread. From these results, at least, it looks like a high concentration of athlete cells seems to deter the growth of even non-local tumor cells.
Disclaimer
This simulation is deliberately simplified to isolate the lactate clearance hypothesis. It could serve as a base model for investigating how additional factors (vascularity, immune response, nutrient competition) interact with this mechanism.
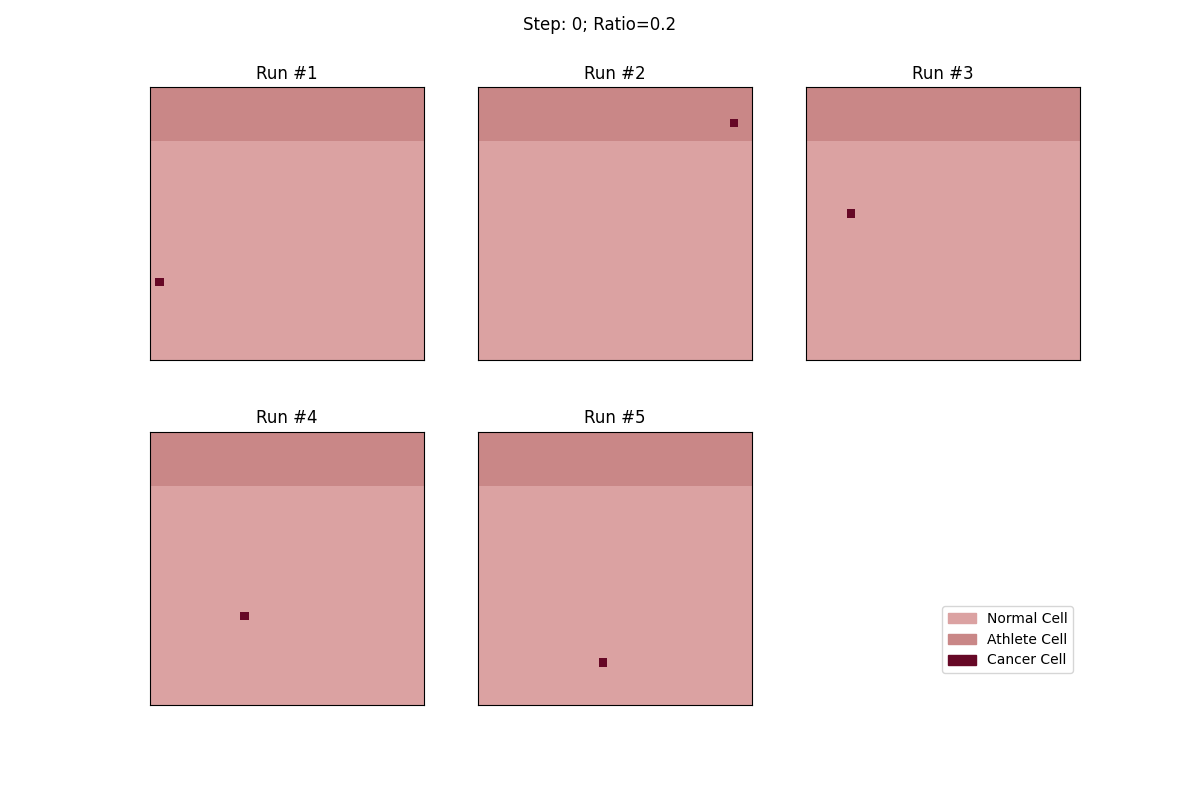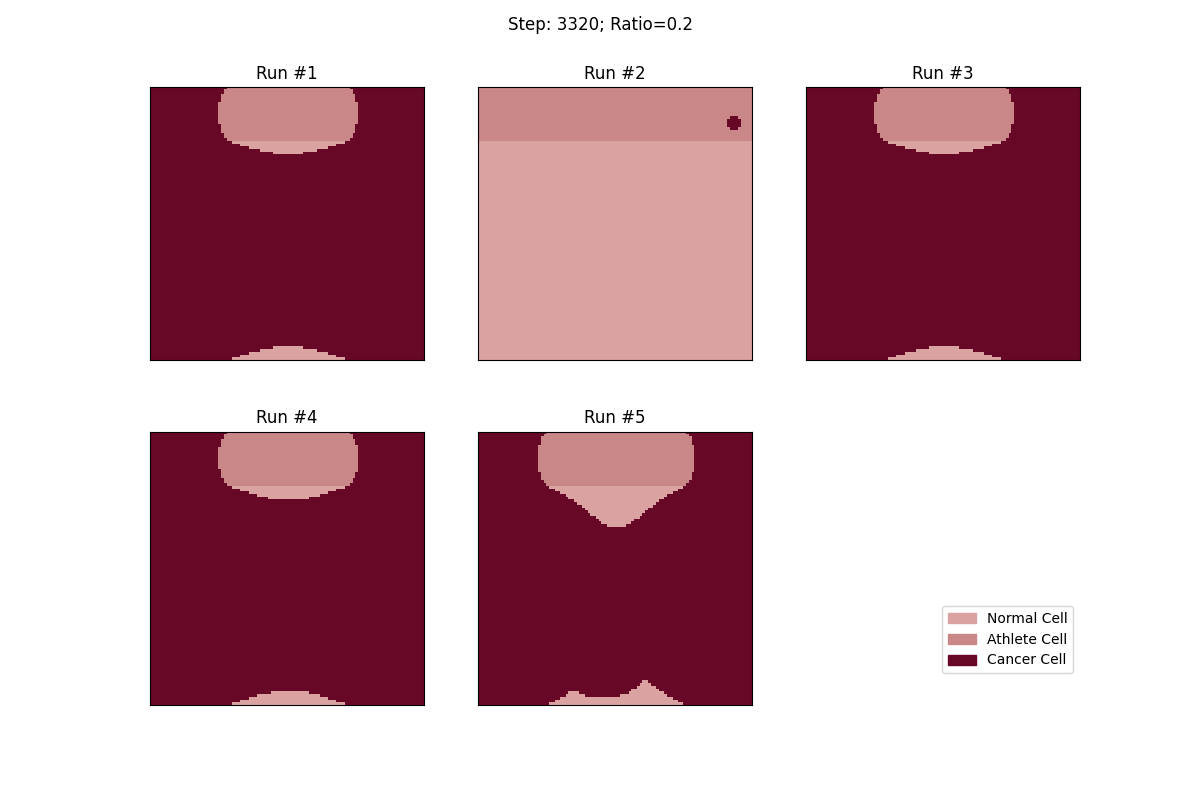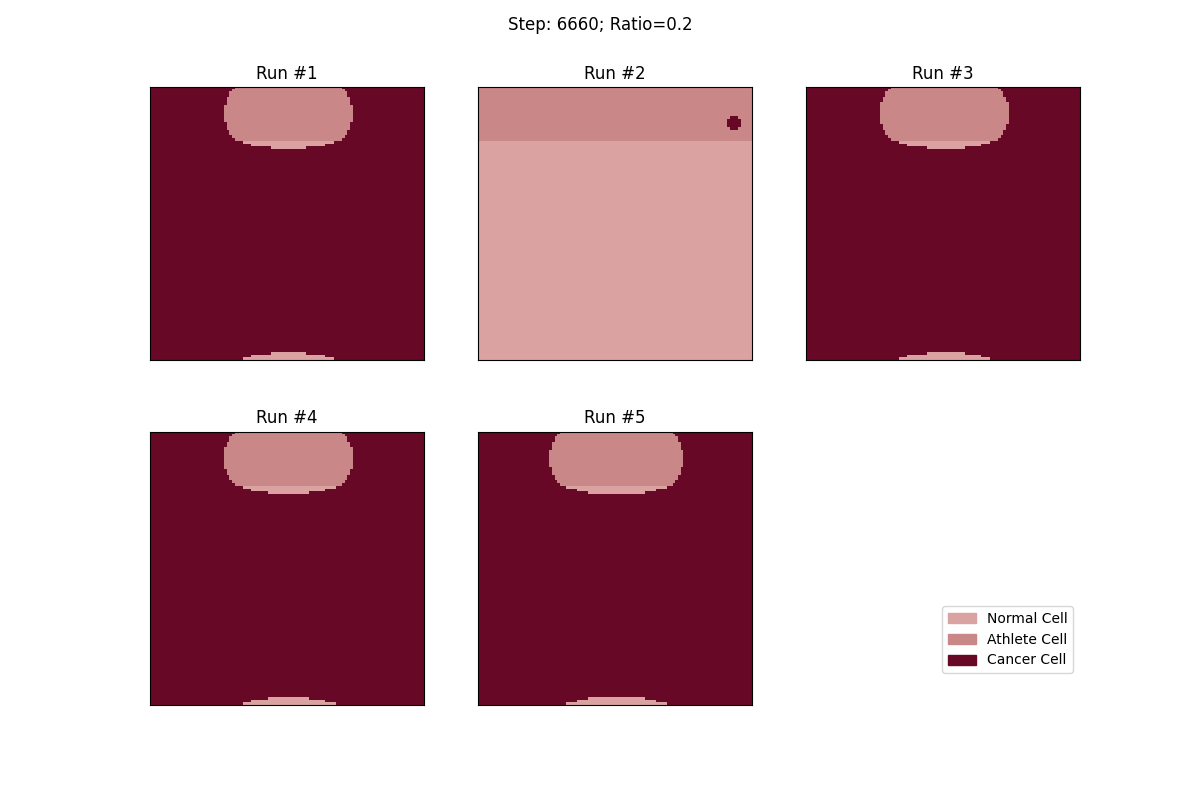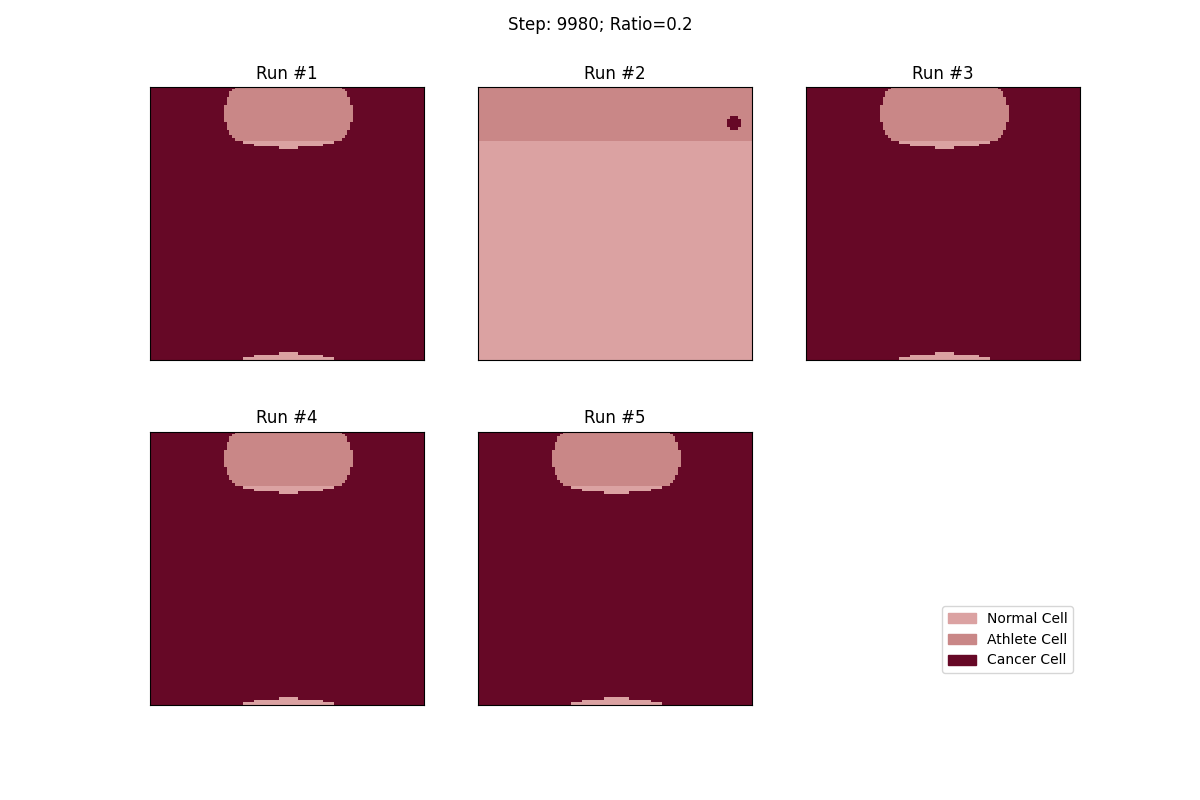
Cellular automata with a 20% ratio of athlete cells. The tumor in the athlete region (Run #2) doesn't spread over time, but all the other tumors, given enough time, eventually spread and take over.
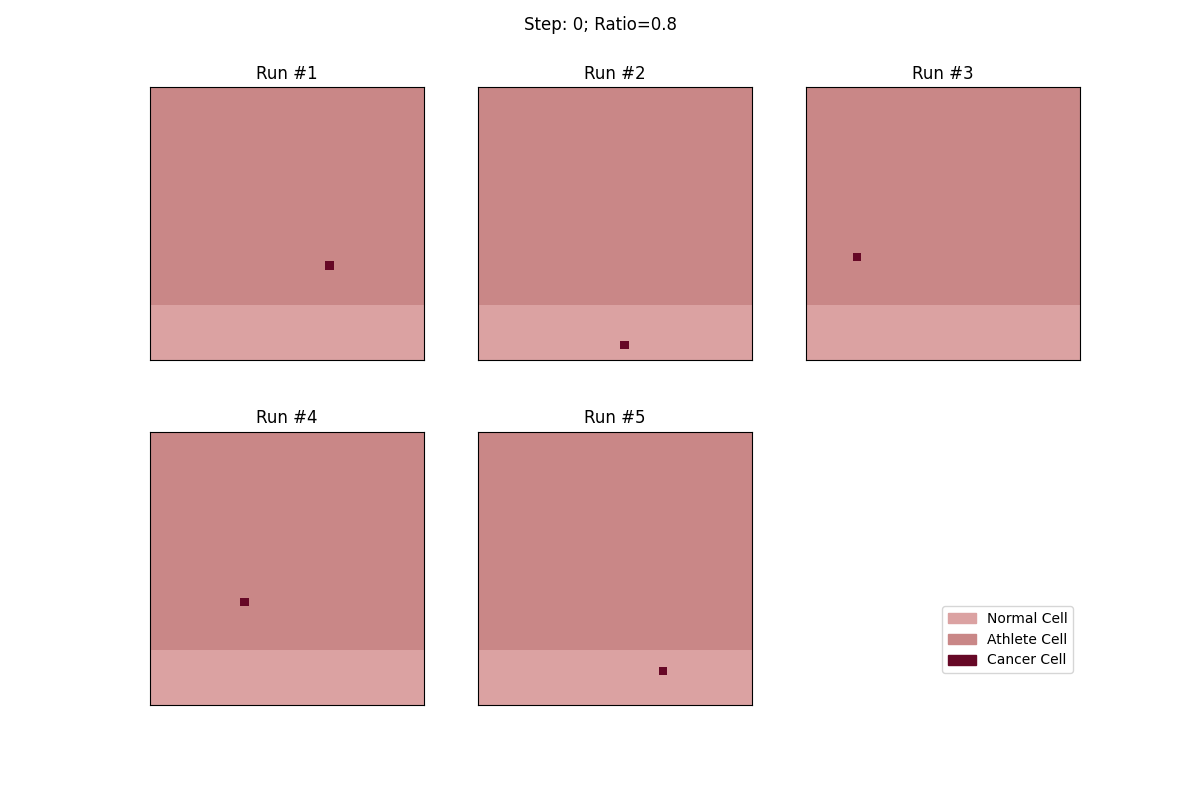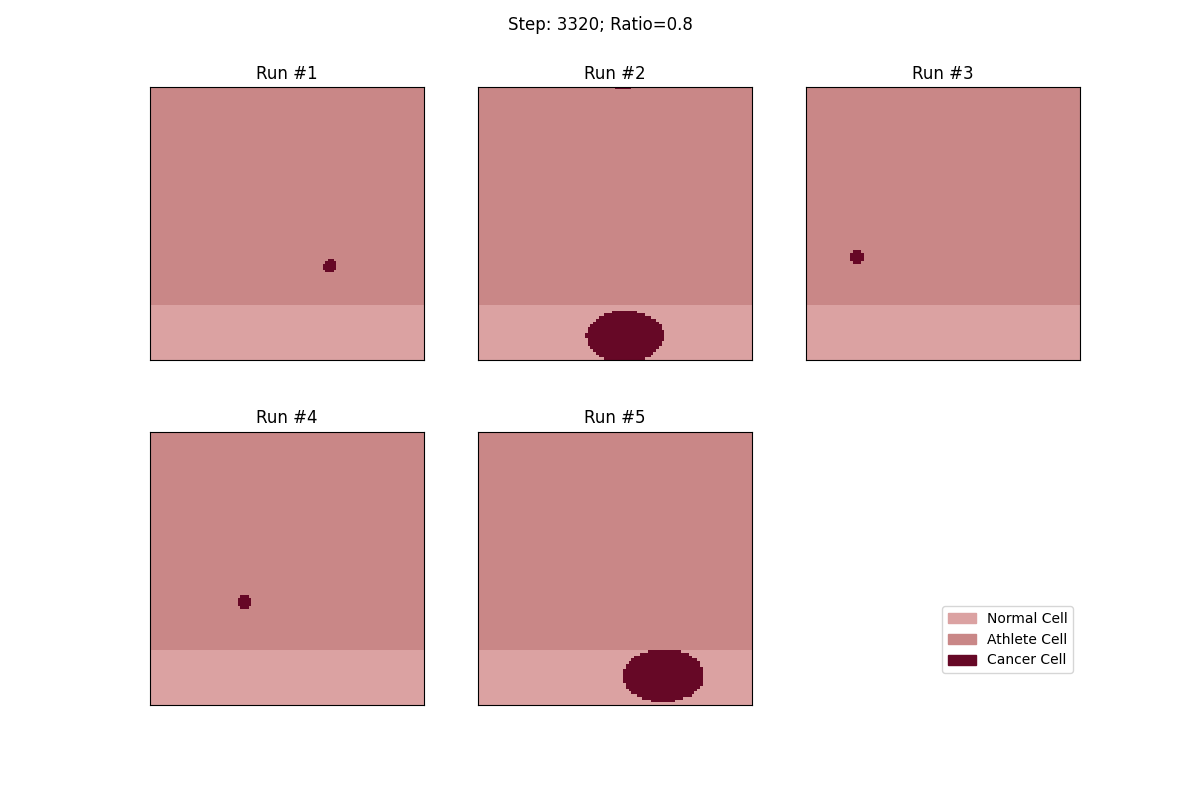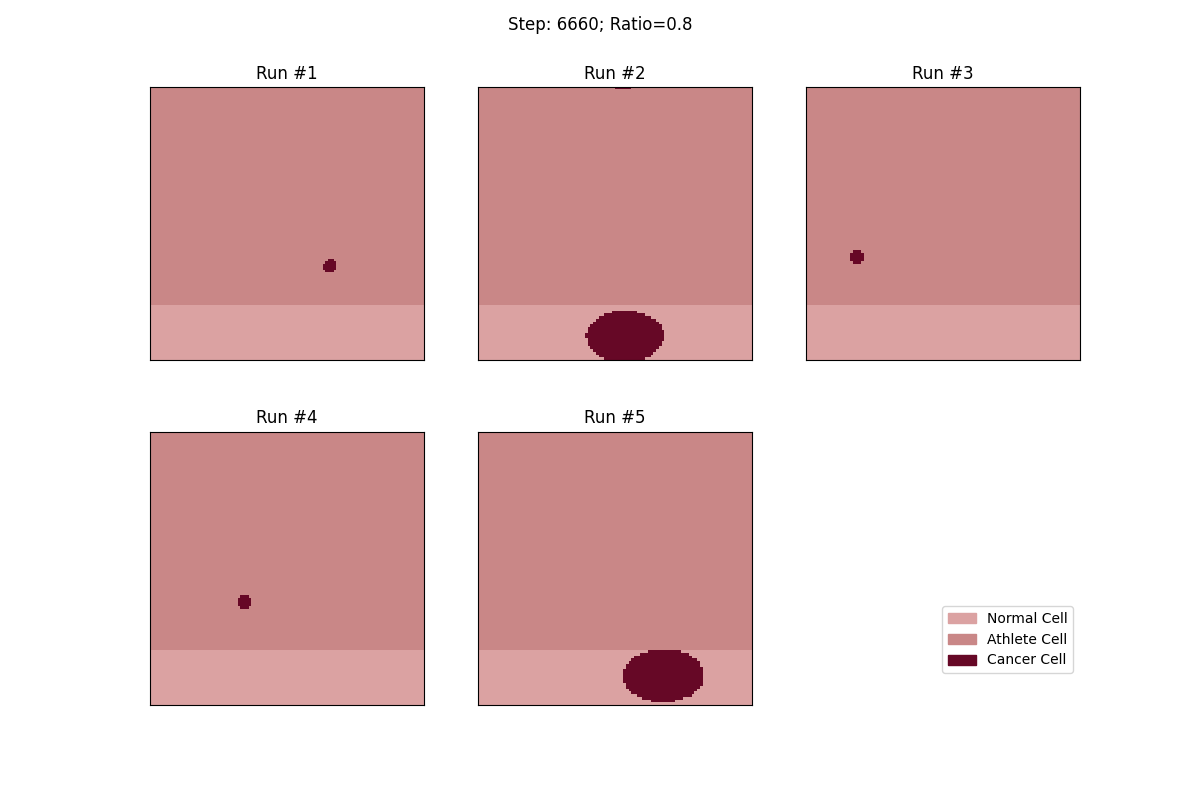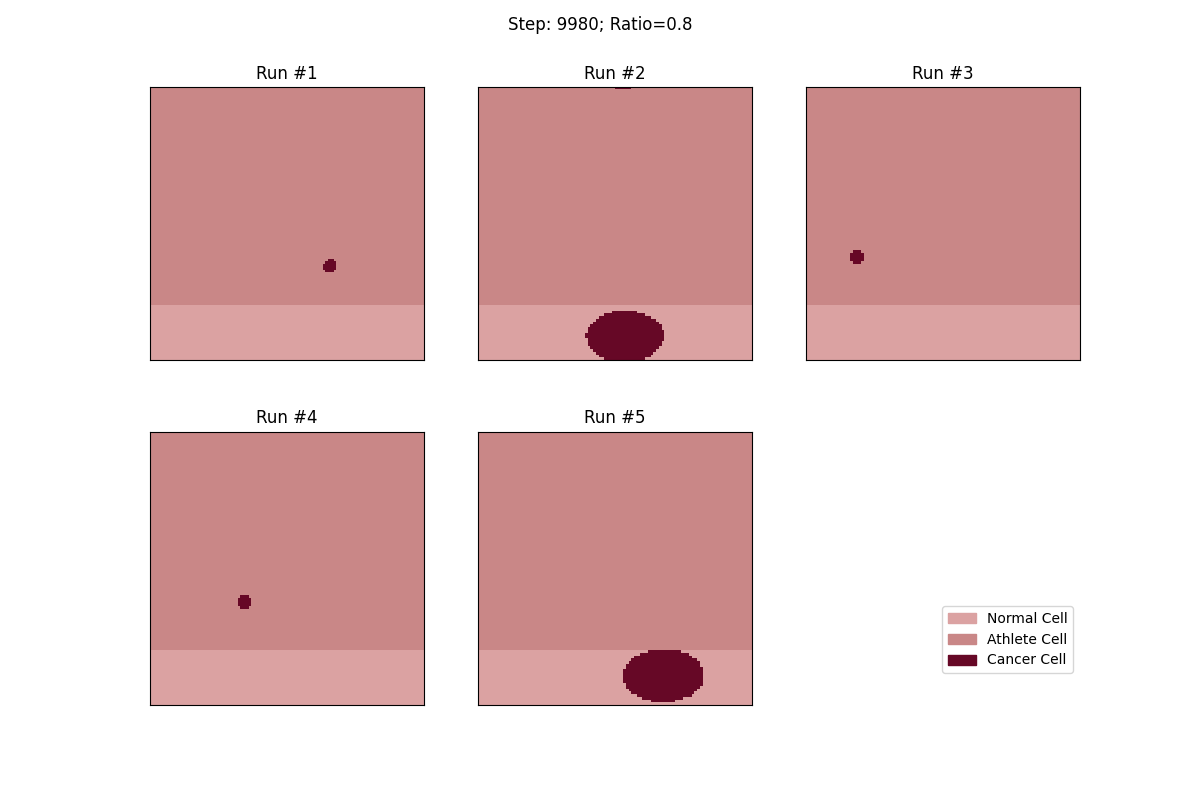
Cellular automata with an 80% ratio of athlete cells. The tumors in these runs have a really hard time growing.